
There are three key elements of a digital clinical trial: recruitment and retention, patient-reported and -collected health data, and digital analytics 18. Here, we describe our experience in the design and execution of this fully remote clinical trial, using digital technologies and infrastructure to provide real-time ascertainment of cardiovascular safety and risk in the evaluation of an investigational drug regimen for COVID-19. In this study, we utilized digital technologies that facilitated collaboration among scientists, implemented internet-based recruitment and enrollment, and leveraged rapid computer-assisted data analysis.
KARDIA MOBILE HEART MONITOR TRIAL
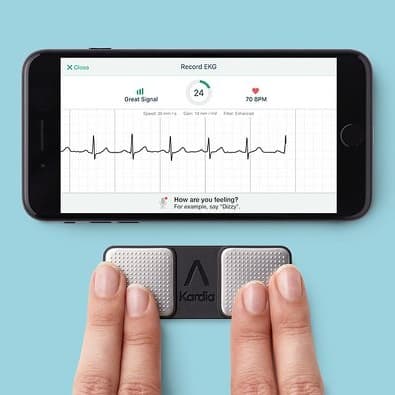
Such remote trials may significantly improve access for patients who live in remote underserved places, work, have children, and/or lack transportation, which are common barriers to trial participation, and thus may help democratize clinical trials. Although hydroxychloroquine has been shown to be ineffective for the treatment of COVID-19 15, 16, 17, our experience with digital cardiac safety monitoring provides important proof of principle for a technology that can be applied broadly to facilitate more pragmatic, “site-less,” and increasingly digital clinical trials of the future. In light of concerns regarding potential cardiovascular toxicities related to hydroxychloroquine- and azithromycin-induced QT prolongation, this trial was designed to rigorously evaluate the cardiovascular safety of these regimens 9, 10, 11, 12, 13, 14. Therefore, we designed a randomized, placebo-equivalent trial to test two therapeutic regimens-hydroxychloroquine and hydroxychloroquine combined with azithromycin-for efficacy in reducing disease progression in ambulatory patients with COVID-19. Hydroxychloroquine and azithromycin, two widely available drugs, were of intense interest at the outset of the pandemic given their potential efficacy against SARS-CoV-2 based on in vitro and early observational data 6, 7, 8. In the context of a critical need to stem the morbidity and mortality of the SARS-CoV-2 pandemic, the development and testing of therapeutics has proceeded at an unprecedented pace 2, 3, 4, 5. Our report demonstrates that digital health technologies can be leveraged to conduct rigorous, safe, and entirely remote clinical trials.Ĭoronavirus disease 2019 (COVID-19), caused by infection with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has spread to more than 194 million people and caused more than 4 million deaths globally as of July 2021 1. QTc prolongation meeting criteria for an adverse event occurred in 28 (12.1%) participants, with 2 occurring in the placebo group, 19 in the hydroxychloroquine group, and 7 in the hydroxychloroquine + azithromycin group. Adherence rates did not differ by age or sex assigned at birth and were high across all reported race and ethnicities. Mean daily adherence to the ECG protocol was 85.2% and was similar to the survey and mid-nasal swab elements of the study. Two hundred and thirty-one participants uploaded 3245 ECGs. ECG collection relied on a consumer device (KardiaMobile 6L, AliveCor Inc.) that recorded and transmitted six-lead ECGs via participants’ internet-enabled devices to a central core laboratory, which measured and reported QTc intervals that were then used to monitor safety. Self-collected vital signs (temperature, respiratory rate, heart rate, and oxygen saturation) and electrocardiographic (ECG) measurements were transmitted digitally to investigators while mid-nasal swabs for SARS-CoV-2 testing were shipped. All study activities were conducted remotely. In this report we describe the implementation of a novel exclusively remote randomized clinical trial ( NCT04354428) of hydroxychloroquine and azithromycin for the treatment of the SARS-CoV-2–mediated COVID-19 disease which included cardiovascular safety monitoring. The coronavirus disease 2019 (COVID-19) pandemic has challenged researchers performing clinical trials to develop innovative approaches to mitigate infectious risk while maintaining rigorous safety monitoring.


 0 kommentar(er)
0 kommentar(er)
